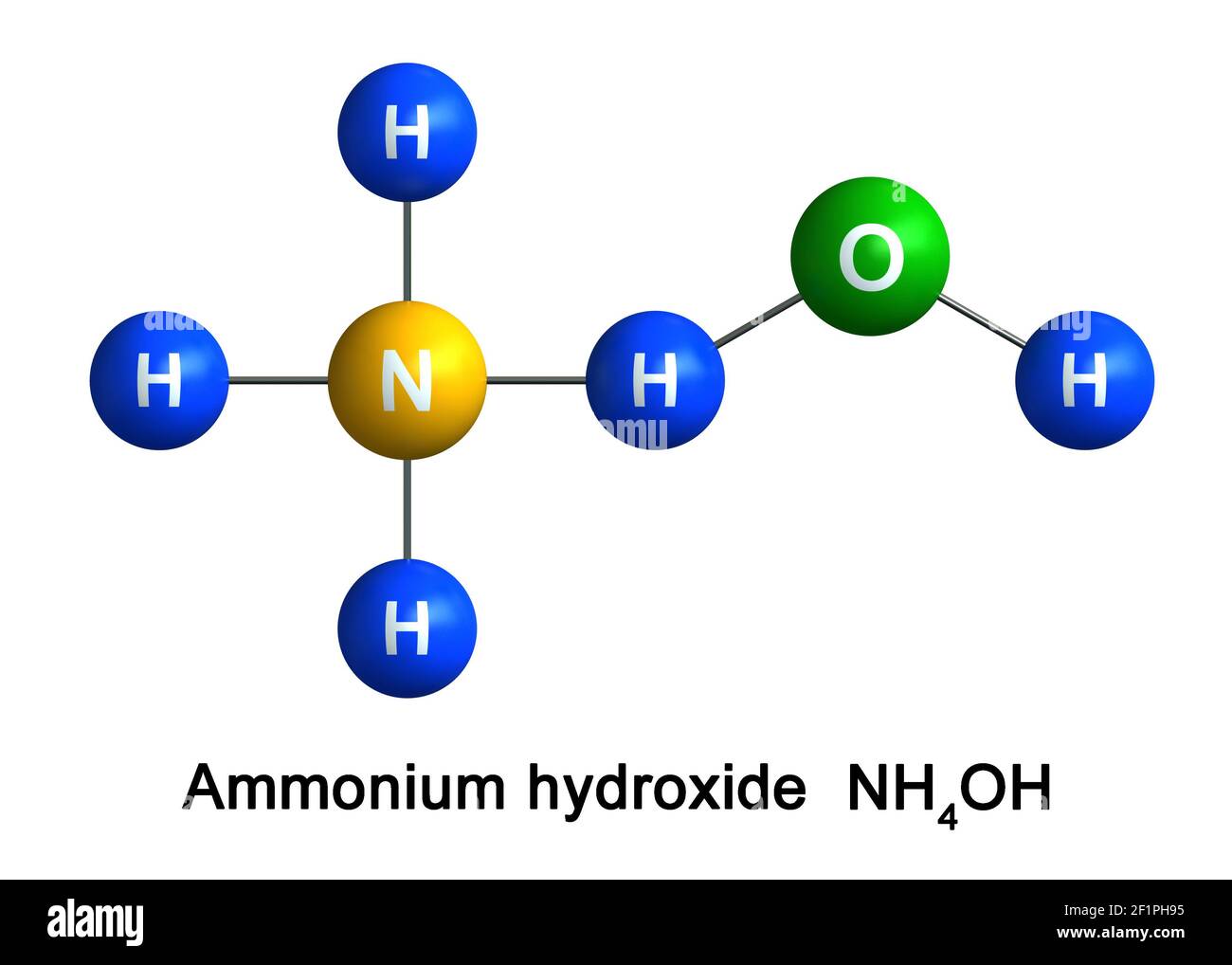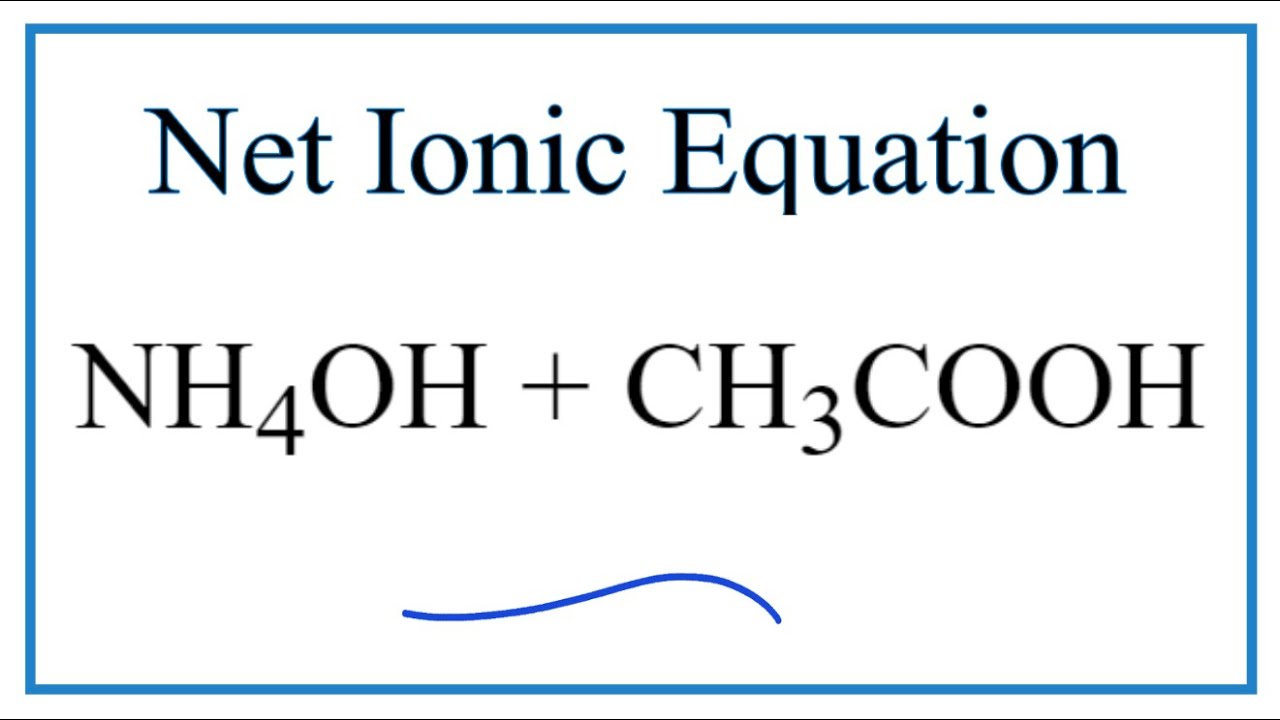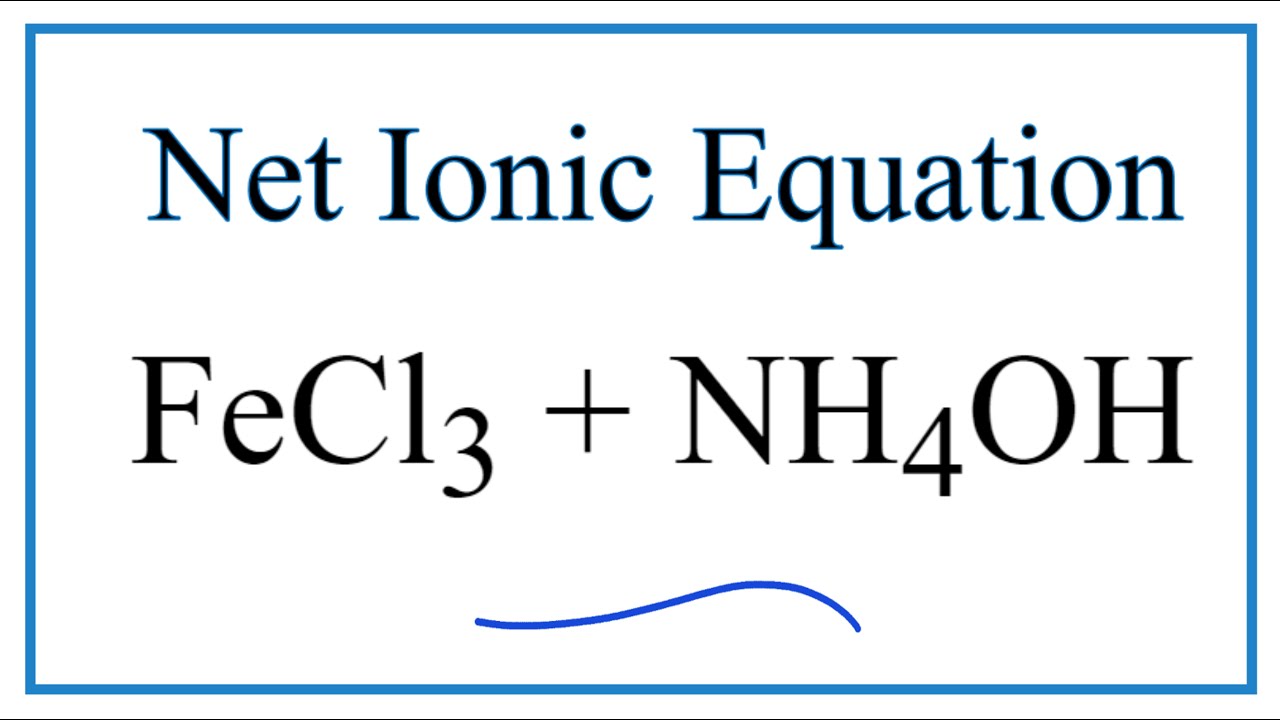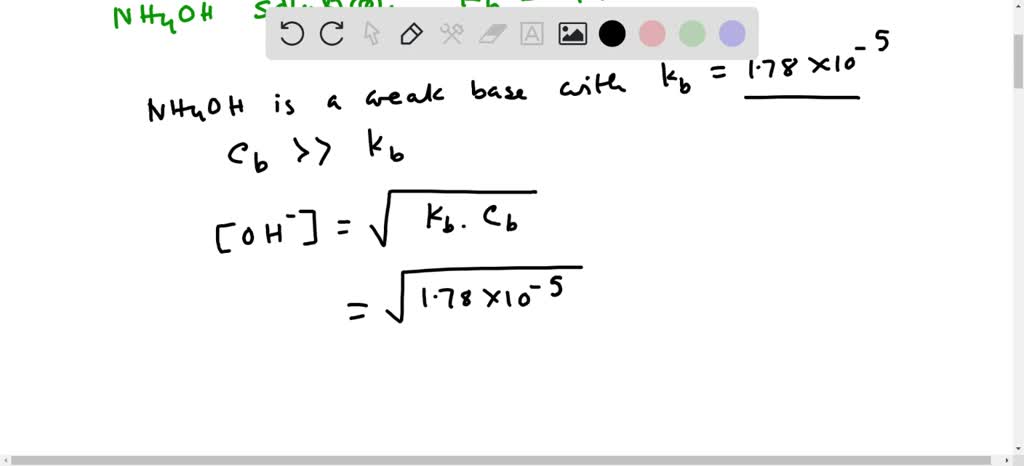
SOLVED: Answer the following equation: Ammonium Hydroxide, NH4OH, is a weak base. Calculate the pH of a 0.60 M solution of ammonium hydroxide. Kb = 1.78 x 10^-5.

The pKb value of ammonium hydroxide is 4.75 An aqueous solution of ammonium hydroxide is titrated with HCl.The pH of the solution at the point where half of the ammonium hydroxide has
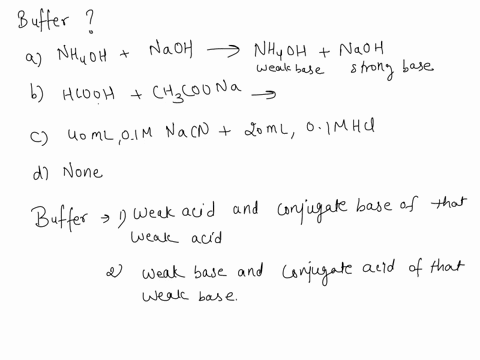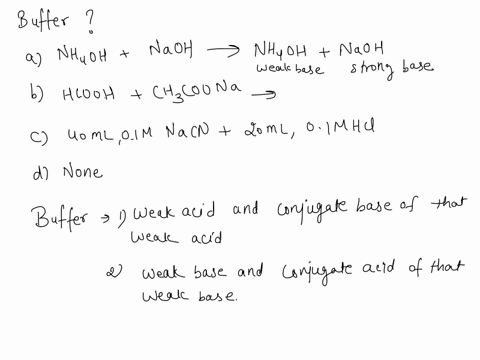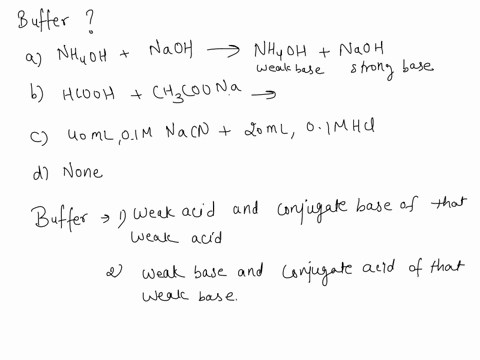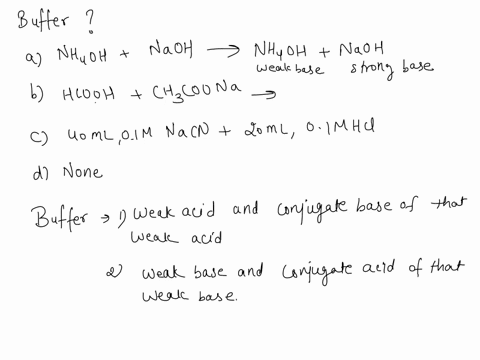

![ANSWERED] When aqueous solutions of NH4OH(aq) and Cu... - Organic Chemistry ANSWERED] When aqueous solutions of NH4OH(aq) and Cu... - Organic Chemistry](https://media.kunduz.com/media/sug-question/raw/52914656-1658983365.4892888.jpeg)